Fundamentals of Alkaline Battery and Carbon Battery
At a time when portable electronics control the market, the selection of the user's battery plays an important role in adapting the use of their things. Today's expanded battery market Technical foundation reflects the unique strength and weaknesses of each battery. To make this article informative, let's start with the universe of the battery corresponding to the user's needs before discussing the difference between alkaline and carbon batteries.
What is a Battery?
A battery is a device that stores chemical energy and converts it into electrical energy to provide a power source for electrical circuits and electronic devices. It typically consists of one or more electrochemical cells, each containing electrodes (anode and cathode) and an electrolyte. When connected to a circuit, a chemical reaction occurs within the battery that generates a flow of electrons, producing an electric current.

Components of Batteries
A battery’s chemical composition means the elements that are used as electrodes (anode and cathode) and the electrolyte that encourage the reactions making electricity. This composition varies depending on the battery type.
Here’s a look at the chemical composition for some common battery types:
1. Alkaline Battery (Primary)
a. Anode (negative electrode) - Zinc (Zn) powder
b. Cathode (positive electrode) - Manganese dioxide (MnO₂)
c. Electrolyte - Potassium hydroxide (KOH) solution (alkaline electrolyte)
2. Carbon Battery (Primary)
a. Anode (negative electrode) - Zinc (Zn) powder
b. Cathode (positive electrode) - Manganese dioxide (MnO₂) mixed with carbon (graphite) as a conductor.
c. Electrolyte - Ammonium chloride (NH₄Cl) or zinc chloride (ZnCl₂) paste.
3. Lead-Acid Battery (Secondary)
a. Anode (negative electrode) - Lead (Pb)
b. Cathode (positive electrode) - Lead dioxide (PbO₂)
c. Electrolyte - Sulfuric Acid (H₂SO₄) solution
4. Lithium-ion Battery (Secondary)
a. Anode (negative electrode) - Graphite (Carbon)
b. Cathode (positive electrode) - Lithium metal oxide
c. Electrolyte - Lithium salt in organic solvent
Let's dive deeply and find out the practicality of carbon battery along with the permanent capacity of alkaline battery.
What is an Alkaline Battery?
Alkaline batteries, the whole term standing for alkaline dry cell or zinc-manganese batteries-are that in between the larger family of zinc-manganese. They have always been the ones with high power output and long life due to their low internal resistance. Since they are mercury-free, these can, therefore, be discarded along with regular trash. Standard in most day-to-day, non-rechargeable batteries found in remotes, flashlights, and toys.
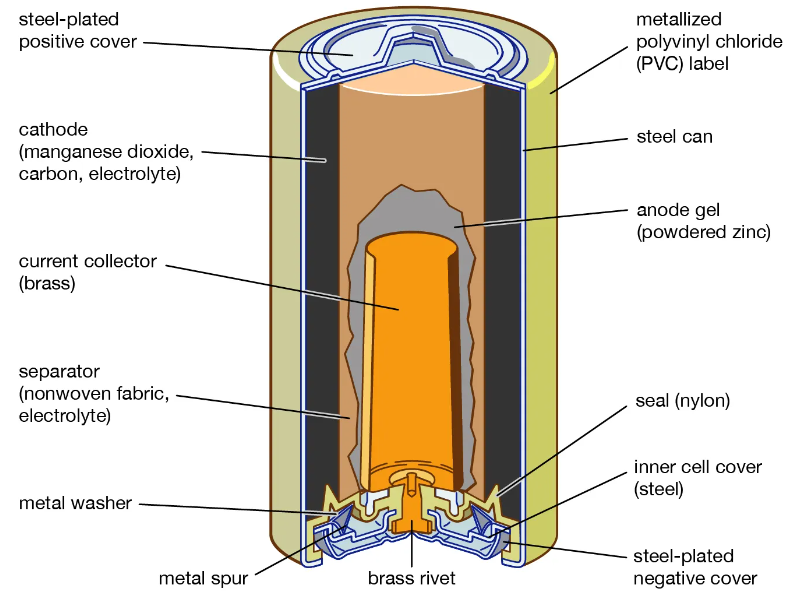
How Does an Alkaline Battery Work?
1. At the Anode (Oxidation): Zinc metal reacts with hydroxide ions from the electrolyte and loses electrons (oxidation reaction). The zinc is converted into zinc oxide or zincate ions.
Chemical Reaction: Zn (s) + 2 OH− (aq) → ZnO (s) + H2O (l) + 2e−
2. Electron Flow: The electrons released at the anode flow through the external circuit towards the cathode.
3. At the Cathode (Reduction): Manganese dioxide reacts with water and electrons to form manganese oxyhydroxide. The cathode (MnO₂) is reduced by gaining electrons (reduction reaction).
Chemical Reaction: 2MnO2(s) + H2O(l) + 2e− → 2MnOOH(s) + 2OH−(aq)
4. Ion Movement in Electrolyte: The electrolyte (KOH) facilitates ion movement inside the battery. The hydroxide ions (OH⁻) move through the electrolyte inside the battery to balance the charges during the reaction.
5. Overall Chemical Reaction in an Alkaline Battery: Zn (s) + 2MnO2(s) → ZnO (s) + Mn2O3(s)
Characteristics of Alkaline Batteries
1. High Energy Density
o They pack more energy into each unit of weight or volume than carbon batteries.
o This results in longer-lasting batteries of the same size.
2. Long Shelf Life
o They keep up to 80-90% of their power after sitting unused for 5-10 years.
o They lose very little charge over time.
3. Stable Voltage Output
o They give out a constant voltage (about 1.5 V per cell) for most of the time they're in use.
o This helps devices work well until the battery is almost dead.
4. Wide Operating Temperature Range
o They function well in temperatures from about -20°C to 54°C.
5. Low Internal Resistance
o Delivers higher current than carbon batteries.
o Works well for devices that need moderate to high power.
6. Non-Rechargeable
o Regular alkaline batteries are primary cells and users cannot recharge them.
7. Safety and Environmental Impact
o Contain no toxic heavy metals like mercury or cadmium.
8. Physical Construction
o Come in cylinder shapes (AA, AAA, C, D sizes).
o Have a zinc powder anode, manganese dioxide cathode, and alkaline KOH electrolyte.
9. Cost-Effective
o Costs more than carbon battery but less than rechargeable lithium-ion batteries.
Advantages of Alkaline Batteries
1. Higher Energy Density:
Alkaline batteries pack more energy than old carbon batteries so they keep going for longer.
2. Long Shelf Life:
These batteries can keep their charge for years (often 5-10 years) without losing much power.
3. Stable Voltage:
Alkaline batteries give out a consistent voltage for most of their life helping devices work more.
4. Widely Available and Inexpensive:
They are mass-produced and commonly available worldwide at a low cost.
5. Non-toxic Materials:
Alkaline batteries score higher when it comes to safety for the environment than mercury batteries did.
Disadvantages of Alkaline Batteries
1. Non-Rechargeable:
Alkaline batteries cannot be recharged once they have been used, even though there are “rechargeable alkaline” varieties and they are less popular among battery users.
2. Environmental Impact:
If thrown into the landfill wrongly, alkaline batteries may cause harm, though they are less toxic than others.
3. Performance Drops in High-Drain Devices:
They are not ideal for devices that require very high currents (like digital cameras or power tools); rechargeable batteries like NiMH or Li-ion perform better there.
4. Not Suitable for Extreme Temperatures:
Performance can degrade in very cold or very hot environments.
Applications of Alkaline Batteries
● Remote Controls (TV, audio equipment), Wireless Computer Peripherals (keyboards, mice), Camera
● Flashlights and Torches, Clocks and Wall Timers, Toys and Portable Games, Personal Care Devices
Pricing and Availability of Alkaline Batteries
Alkaline Batteries can be purchased from the website of unikeyic Electronics. 9V Alkaline zinc manganese dioxide battery (PX1604) is available for a price of $21.46. 1.5V Alkaline zinc manganese dioxide battery (PX1300) is available for a price of $28.38 (1 Qty).

PX1604 and PX1300
What is a Carbon Battery?
A carbon battery, also known as a zinc-carbon battery, which is one of the oldest and simplest kinds of a non-rechargeable or, as termed, primary battery. It is still used in low-drain devices as it is inexpensive. Zinc-carbon or dry cells are known as such because one of Leclanché’s great improvements to the battery was replacing a liquid electrolyte with a paste; these cells got their popular name from their inventor.
There are two basic forms of zinc–carbon dry cells, cylindrical cells and flat cells. Cells sold as cylindrical cells are sold either as single cells or in a pack of two or three, whereas those sold as flat cells are packed in more than four to 300 cells.
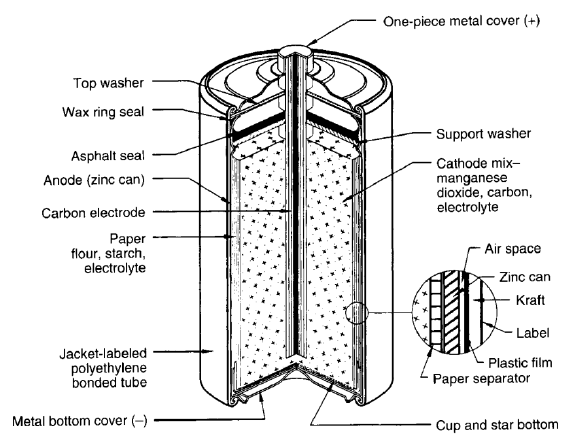
How Does a Carbon Battery Work?
1. At the Anode (Oxidation): Zinc metal loses electrons and is oxidized into zinc ions.
Chemical Reaction: Zn (s) → Zn2+ + 2e−
2. Electron Flow: The electrons flow through the external circuit is responsible for powering the device.
3. At the Cathode (Reduction): Manganese dioxide gains electrons and reacts with ammonium ions to complete the reaction.
4. Chemical Reaction: 2MnO2 + 2NH4+ + 2e− → Mn2O3 + 2NH3 + H2O
5. Ion Movement: Ions move through the electrolyte to maintain charge balance.
Characteristics of Carbon Batteries
1. Low Energy Density
o Store less energy per unit weight or volume compared to alkaline batteries.
2. Shorter Shelf Life
o Typically, last 1 to 3 years. Higher self-discharge rate compared to alkaline batteries.
3. Voltage Output
o Nominal voltage of about 1.5 V per cell, similar to alkaline batteries.
o However, voltage drops faster under load due to higher internal resistance.
4. Higher Internal Resistance
o Results in poor performance under high current or heavy load conditions.
5. Leakage Risk
o More prone to leakage after prolonged use or storage, which can damage devices.
6. Cost-Effective
o Generally cheaper than alkaline batteries, making them attractive for low-drain applications.
7. Non-Rechargeable
o Zinc-carbon batteries are primary cells and cannot be recharged.
8. Environmental Considerations
o Safer than older mercury-based batteries but less environmentally friendly than some modern batteries.
9. Physical Construction
o Typically, cylindrical with a zinc can anode, MnO2 cathode, and NH4Cl or ZnCl2 paste electrolyte.
Advantages of Carbon Batteries
● Very inexpensive to manufacture.
● Easy to produce and widely available.
● Works well for low-drain devices that don’t need a continuous power supply.
Disadvantages of Carbon Batteries
● Provides less power and shorter lifespan compared to alkaline batteries.
● Higher internal resistance with shorter shelf life.
● Poor performance with high-drain devices like digital cameras or power tools.
Applications of Carbon Batteries
● Flashlights (low-drain types), Clocks and small toys.
● Remote controls (low power), Transistor radios (older models), Portable flashlights.

1.5V Carbon Zinc Battery
Pricing and Availability of Carbon Batteries
Carbon Batteries can be purchased from the website of Unikeyic Electronics. 1.5V Carbon Zinc Battery (Part Number: 13331-0001) is available for a price of $4.89. 1.3V Carbon Zinc Battery (Part Number: 13299-0001) is available for a price of $6.23.

3V Carbon zinc battery (13299-0002)
Related Recommended Reading:
AG10 Battery: Exploring the Fascinating World and Applications of Miniature Energy